Synthesis of Some New 1,3,4- Oxadiazole Derivatives and Thiazolidine Derived from Cysteine and Evaluation their Anticancer (MCF7) Activity
DOI:
https://doi.org/10.48112/bcs.v1i2.109Abstract
 Abstract Views: 221
Abstract Views: 221
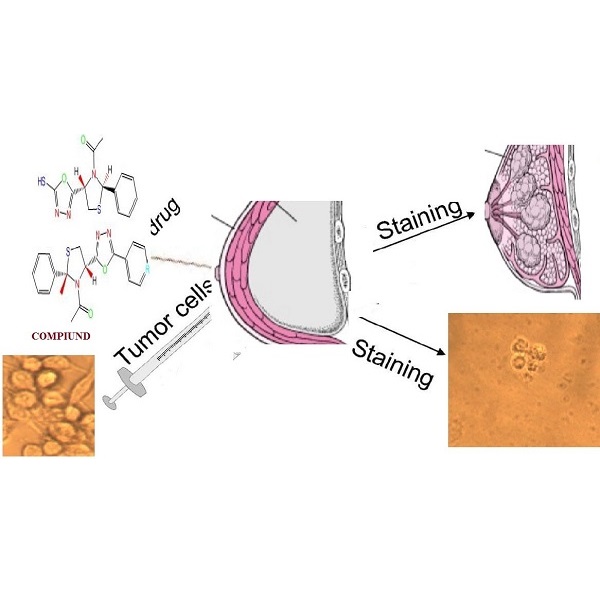
The study focused on the preparation of oxadiazole derivatives containing thiazolidine. Thiazolidine firstly was prepared from the reaction of benzaldehyde with L-cysteine with a good yield and then it was reacted with acetic anhydride to prepare acetyl thiazolidine, then with ethanol in the presence of H2SO4, then steps were taken to prepare a thiazolidine hydrazide (A3), which was reacted with aromatic carboxylic acid in presence POCl3 or carbon disulphide and base KOH to obtain oxadiazole derivatives A4-8. These compounds characterized using FT-IR, NMR and Mass (EI) were diagnosed and the synthesized compound were validated. The activity of oxadiazole derivatives were studied against breast cancer cells, the two compound A8, A4 showed good activity against the cells as for the compounds A5, A6, A7 it was showed little activity against these cells and the value IC50 was calculated.
Keywords:
Anticancer MCF-7, L-cysteine, Oxadiazole, ThiazolidinesMetrics
References
Ahsan, M. J. (2018). Synthesis and cytotoxicity evaluation of [(2, 4-dichlorophenoxy) methyl]-5-aryl-1, 3, 4-oxadiazole/4$ H $-1, 2, 4-triazole analogues. Turkish Journal of Chemistry, 42(5), 1334-1343.
Al-Badrany, K. A., Mohammed, A. S., & Alasadi, Y. K. (2019). Synthesis of some new 1, 3, 4-oxadiazole compounds derived from 1H-imidazole and study their biological activity. Eurasian Journal of Biosciences, 13(1), 501-507.
Bondock, S., Adel, S., Etman, H. A., & Badria, F. A. (2012). Synthesis and antitumor evaluation of some new 1, 3, 4-oxadiazole-based heterocycles. European Journal of Medicinal Chemistry, 48, 192-199. https://doi.org/10.1016/j.ejmech.2011.12.013
Busacca, C. A., Dong, Y., & Spinelli, E. M. (1996). A one step synthesis of thiazolines from esters. Tetrahedron letters, 37(17), 2935-2938. https://doi.org/10.1016/0040-4039(96)00451-0
Da Silva, T. L., Miolo, L. M. F., Sousa, F. S., Brod, L. M., Savegnago, L., & Schneider, P. H. (2015). New thioureas based on thiazolidines with antioxidant potential. Tetrahedron Letters, 56(48), 6674-6680. https://doi.org/10.1016/j.tetlet.2015.10.037
El-Sharkawy, K. A. (2011). Synthesis and Antimicrobial Activity of 2-Substituted-3-Acetyl Thiazolidine-4-Carbonyl-Amino acid Derivatives. Journal of Pharmaceutical Sciences and Research, 3(1), 1005-1014.
Gilani, S. J., Khan, S. A., & Siddiqui, N. (2010). Synthesis and pharmacological evaluation of condensed heterocyclic 6-substituted 1, 2, 4-triazolo-[3, 4-b]-1, 3, 4-thiadiazole and 1, 3, 4-oxadiazole derivatives of isoniazid. Bioorganic & Medicinal Chemistry Letters, 20(16), 4762-4765. https://doi.org/10.1016/j.bmcl.2010.06.125
Guessas, B., Othman, A. A., & Khiati, Z. (2007). Synthesis and antibacterial activity of 1, 3, 4-oxadiazole and 1, 2, 4-triazole derivatives of salicylic acid and its synthetic intermediates. South African Journal of Chemistry, 60(1), 20-24. https://hdl.handle.net/10520/EJC24423
Husain, A., Rashid, M., Mishra, R., Parveen, S., Shin, D. S., & Kumar, D. (2012). Benzimidazole bearing oxadiazole and triazolo-thiadiazoles nucleus: Design and synthesis as anticancer agents. Bioorganic & medicinal chemistry letters, 22(17), 5438-5444. https://doi.org/10.1016/j.bmcl.2012.07.038
Jafari, E., Mohammadi, T., Jahanian-Najafabadi, A., & Hassanzadeh, F. (2017). Synthesis and antimicrobial evaluation of some 2, 5 disubstituted 1, 3, 4-oxadiazole derivatives. Research in pharmaceutical sciences, 12(4), 330. https://dx.doi.org/10.4103%2F1735-5362.212051
Leod, G. M., & Ames, J. M. (1987). Effect of water on the production of cooked beef aroma compounds. Journal of Food Science, 52(1), 42-45. https://doi.org/10.1111/j.1365-2621.1987.tb13968.x
Majed, A. A., & Abid, D. S. (2015). Synthesis, Characterization and Biological Activity Study of some New Thiazolidine Derivatives. Basrah Journal of Science (C), 33(1), 101-117.
Majed, A. A., & Abid, D. S. (2021). Synthesis, Characterization and Biological Activity and Anti-Oxidant Study of Some New Thiazolidine Derivatives Containing Oxadiazole. Annals of the Romanian Society for Cell Biology, 11935-11945.
Mohammed, M. A., Abid, D. S., & Sayyah, S. G. (2021). Preparation, Characterization of some Thiazolidine Derived from Penicillamine and their Antioxidants Activity. Annals of the Romanian Society for Cell Biology, 4653-4663.
Nawar, F., Al-Asadi, R., & Abid, D. (2020). Synthesis, Antibacterial Activity and DFT Calculations of Some Thiazolidine-4-Carboxylic acid Derivatives and Their Complexes with Cu (II), Fe (II) and VO (II). Egyptian Journal of Chemistry, 63(1), 349-362. https://dx.doi.org/10.21608/ejchem.2019.16096.1986
Özyazici, T., Şahin, F., & Akkoç, M. K. (2021). Synthesis, spectral characterization, and biological studies of 3, 5-disubstituted-1, 3, 4-oxadiazole-2 (3H)-thione derivatives. Turkish Journal of Chemistry, 45(3), 749-760.
Pavin, N. F., Donato, F., Cibin, F. W., Jesse, C. R., Schneider, P. H., de Salles, H. D., ... & Savegnago, L. (2011). Antinociceptive and anti-hypernociceptive effects of Se-phenyl thiazolidine-4-carboselenoate in mice. European journal of pharmacology, 668(1-2), 169-176. https://doi.org/10.1016/j.ejphar.2011.06.038
Pitasse-Santos, P., Sueth-Santiago, V., & Lima, M. E. (2018). 1, 2, 4-and 1, 3, 4-Oxadiazoles as Scaffolds in the Development of Antiparasitic Agents. Journal of the Brazilian Chemical Society, 29, 435-456. https://doi.org/10.21577/0103-5053.20170208
Şahin, G., Palaska, E., Ekizoğlu, M., & Özalp, M. (2002). Synthesis and antimicrobial activity of some 1, 3, 4-oxadiazole derivatives. Il Farmaco, 57(7), 539-542. https://doi.org/10.1016/S0014-827X(02)01245-4
Šubr, V., & Ulbrich, K. (2006). Synthesis and properties of new N-(2-hydroxypropyl) methacrylamide copolymers containing thiazolidine-2-thione reactive groups. Reactive and Functional Polymers, 66(12), 1525-1538. https://doi.org/10.1016/j.reactfunctpolym.2006.05.002
Taha, M., Ismail, N. H., Imran, S., Wadood, A., Rahim, F., Saad, S. M., ... & Nasir, A. (2016). Synthesis, molecular docking and α-glucosidase inhibition of 5-aryl-2-(6′-nitrobenzofuran-2′-yl)-1, 3, 4-oxadiazoles. Bioorganic chemistry, 66, 117-123. https://doi.org/10.1016/j.bioorg.2016.04.006
Tawfiq, M. T. (2016). Synthesis and Characterization of Some New 4-Oxothiazolidine-2-Carboxylic Acid Derivatives With the Evaluation of Their Biological Activity. Diyala J. For PureSci, 12, 43-64.
Wang, Z., Wang, M., Yao, X., Li, Y., Qiao, W., Geng, Y., ... & Wang, Q. (2012). Hydroxyl may not be indispensable for raltegravir: Design, synthesis and SAR studies of raltegravir derivatives as HIV-1 inhibitors. European journal of medicinal chemistry, 50, 361-369. https://doi.org/10.1016/j.ejmech.2012.02.015
Zhang, M. Z., Mulholland, N., Beattie, D., Irwin, D., Gu, Y. C., Chen, Q., ... & Clough, J. (2013). Synthesis and antifungal activity of 3-(1, 3, 4-oxadiazol-5-yl)-indoles and 3-(1, 3, 4-oxadiazol-5-yl) methyl-indoles. European Journal of Medicinal Chemistry, 63, 22-32. https://doi.org/10.1016/j.ejmech.2013.01.038
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Biomedicine and Chemical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.


















